
Digital Stethoscope in the Era of Artificial Intelligence: A Comprehensive Review in the Era of Evidence-based Clinical Studies
Artificial Intelligence受け取った 17 Oct 2025 受け入れられた 07 Nov 2025 オンラインで公開された 08 Nov 2025
ISSN: 2995-8067 | Quick Google Scholar
Next Full Text
Effect of Additive Manufacturing Parameters on 316L Mechanical and Corrosion Behavior


受け取った 17 Oct 2025 受け入れられた 07 Nov 2025 オンラインで公開された 08 Nov 2025
Digital stethoscopes, combined with artificial intelligence (AI), are transforming clinical auscultation across pediatrics, cardiology, and pulmonology. This review summarizes recent evidence-based studies evaluating AI-assisted digital stethoscopes for the detection and classification of heart and lung sounds. Data indicate improved diagnostic accuracy, enhanced objectivity, and potential applications in low-resource and telemedicine settings. Meta-analysis of key studies shows significant increases in sensitivity, specificity, and AUC compared with traditional auscultation. Future perspectives include wearable devices, multimodal diagnostics, personalized sound profiling, augmented reality integration, and interoperability within the Internet of Medical Things (IoMT).
The stethoscope, since its invention in 1816, has remained a symbol of medical practice and a fundamental diagnostic tool. Despite technological advancements, conventional auscultation relies heavily on clinician skill, experience, and auditory acuity. This subjectivity can result in misclassification of heart murmurs, delayed detection of pulmonary abnormalities, and missed early signs of cardiovascular compromise [].
Digital stethoscopes have emerged as a technological solution, converting acoustic signals into electronic data that can be amplified, filtered, and visualized. When integrated with artificial intelligence (AI), these devices enable automated sound classification, predictive analytics, and continuous monitoring. AI algorithms can identify subtle patterns beyond human auditory perception, offering objective assessments and potentially improving patient outcomes [].
Evidence-based medicine supports the adoption of AI-enhanced digital stethoscopes. Multiple clinical studies demonstrate their superior performance in detecting pediatric heart murmurs, neonatal respiratory distress, valvular heart disease, and chronic pulmonary conditions. Additionally, these devices offer cost-effective, non-invasive solutions suitable for low-resource settings and telemedicine [].
This review examines the current clinical evidence, technological innovations, and future directions of AI-integrated digital stethoscopes.
A systematic literature search was performed in PubMed, Scopus, and Web of Science from 2010 to 2025 using terms such as “digital stethoscope,” “artificial intelligence,” “machine learning,” “heart murmur,” and “respiratory auscultation.” Reference lists of relevant articles were also screened.
Eligible studies included prospective, comparative, and randomized clinical trials evaluating digital stethoscopes with or without AI integration. Studies were selected if they: Focused on cardiology, pulmonology, or pediatric populations; Reported objective diagnostic outcomes (sensitivity, specificity, accuracy, AUC).
Data extracted included study population, technology used, clinical focus, and diagnostic performance. Two reviewers independently extracted data, resolving disagreements by consensus. Results were summarized narratively and in tables, emphasizing AI-assisted versus conventional auscultation.
As a literature-based review, no ethical approval was required.
Digital stethoscopes convert mechanical vibrations into electronic signals using microphones or piezoelectric sensors. These signals can then be amplified, filtered, and analyzed in real time. AI integration enhances the device's functionality, enabling pattern recognition, classification of heart and lung sounds, and predictive modeling [].
Key technological components include:
− Signal processing: Noise reduction, amplification, and waveform visualization improve diagnostic fidelity.
− Deep learning algorithms: Convolutional neural networks (CNNs) and long short-term memory (LSTM) networks enable multi-class sound classification and predictive analysis.
− Connectivity: Integration with mobile devices, cloud platforms, and electronic health records (EHRs) facilitates telemedicine, longitudinal monitoring, and remote consultation.
The combination of high-fidelity signal acquisition and AI-driven analysis transforms auscultation from a subjective skill into an objective, reproducible diagnostic process. This capability is particularly relevant in specific patient populations (e.g., pediatric and neonatal populations), in specific medical contexts (e.g., in emergency and reanimation), where subtle physiological variations may be clinically significant but difficult to detect with conventional stethoscopes.
Heart murmurs are common in children, often benign but occasionally indicative of structural heart disease. Traditional auscultation relies heavily on clinician experience, leading to misclassification. Zhou et al. [] demonstrated a deep learning algorithm capable of multi-class classification of pediatric heart sounds using digital stethoscopes. The study reported AUC values of 0.92, 0.83, and 0.88 for normal heart sounds, innocent murmurs, and pathological murmurs, respectively. AI integration provides a reliable, objective tool for murmur classification, supporting early diagnosis, clinical decision-making, and parental reassurance.
These AI-powered stethoscopes also foster clinician confidence by reducing reliance on subjective judgment and providing a quantifiable assessment of heart sounds. Their adoption in pediatric cardiology could standardize evaluations, facilitate early interventions, and reduce unnecessary referrals.
Table 1 includes all recent studies on digital stethoscopes in Cardiology, Pulmonology, and Pediatrics (Table 1).
Respiratory conditions such as asthma, bronchiolitis, and pneumonia remain leading causes of morbidity in children. Accurate auscultation is essential but often limited by the subtlety of abnormal sounds and patient cooperation. Kevat, et al. [] reported that digital stethoscopes offered higher sensitivity than clinician auscultation for wheezes and achieved 100% concordance for crackle detection. Zhou, et al.
[] further demonstrated superior detection of wheezes, crackles, and stridor compared to traditional auscultation.
Digital stethoscopes enable objective analysis of sound characteristics, including waveform periodicity and frequency. This capability improves early identification of respiratory distress, guiding timely interventions and reducing the risk of complications. The technology is particularly valuable for pediatric patients who may struggle to verbalize symptoms.
Diagnosing pneumonia in resource-limited environments is challenging due to limited access to radiological imaging. The Pneumonia Etiology Research for Child Health (PERCH) study [] analyzed over 1,000 lung sound recordings in children aged 1–59 months. Digital stethoscopes reliably captured crackles and wheezes, with a higher prevalence of abnormal lung sounds in children with severe pneumonia compared to healthy controls.
This evidence underscores the potential of digital stethoscopes as non-invasive, cost-effective diagnostic tools, improving early detection and treatment planning in low-resource settings where conventional imaging may be unavailable.
Early recognition of neonatal respiratory distress is critical for preventing severe complications. Grooby, et al. [] applied a Random Undersampling Boosting (RUSBoost) classifier to digital stethoscope recordings in term newborns. The model achieved specificity of 85%, sensitivity of 66.7%, and overall accuracy of 81.8%.
AI-assisted digital auscultation provides objective data to inform early interventions in neonatal care. This approach is especially valuable in intensive care settings, enabling clinicians to monitor subtle changes in respiratory function and act promptly.
Valvular heart disease is a leading cause of morbidity and mortality, yet early detection is often limited by subjective auscultation. Chorba, et al. [] trained a deep learning algorithm on over 34 hours of annotated heart sounds. The model demonstrated a sensitivity of 76.3% and specificity of 91.4% for murmurs, improving to 90% sensitivity when softer murmurs were excluded.
Detection of moderate-to-severe aortic stenosis reached 93.2% sensitivity and mitral regurgitation 94.6% specificity, highlighting AI-powered stethoscopes as potential screening tools. By reducing reliance on echocardiography, these devices could lower costs, improve access to care, and support early intervention.
Peripartum cardiomyopathy (PPCM) affects women during late pregnancy and postpartum, with potentially fatal outcomes. In Nigeria, Adedinsewo, et al. [] conducted a randomized trial involving 1,232 women. AI-guided digital stethoscopes identified left ventricular systolic dysfunction (LVSD) in 4.1% of participants, nearly doubling the detection rate of the control group (2.0%).
These findings demonstrate the feasibility of AI-assisted screening in obstetric populations, particularly in areas with limited access to echocardiography, enabling earlier diagnosis and timely management.
Guo, et al. [] evaluated the integration of digital stethoscopes with single-lead ECGs for detecting asymptomatic left ventricular systolic dysfunction. Using a convolutional neural network (CNN), the model achieved AUC 0.85, sensitivity 77.5%, and specificity 78.3%.
This approach highlights the potential of combined auscultation and ECG analysis for early identification of heart failure. Non-invasive, scalable screening may improve patient outcomes and reduce healthcare costs by facilitating timely intervention.
Digital stethoscopes, combined with AI, offer innovative approaches for diagnosing chronic pulmonary conditions such as interstitial lung disease (ILD) and chronic obstructive pulmonary disease (COPD). Siebert, et al. [] collected lung sounds from 10 thoracic sites, integrating data with lung ultrasound imaging. Deep learning models, including CNNs and LSTM networks, achieved AUC >80%, suggesting potential for precise diagnosis, stratification, and monitoring of chronic respiratory diseases.
During the COVID-19 pandemic, AI-powered digital stethoscopes facilitated rapid and consistent diagnosis. Glangetas, et al. [] demonstrated the capability to predict disease severity based on lung sound analysis, enhancing triage and management in decentralized and telemedicine settings.
This application highlights the potential for AI-enhanced auscultation to support pandemic response, particularly in resource-limited environments lacking access to imaging or laboratory diagnostics.
Across pediatric, cardiac, and pulmonary populations, AI-assisted digital stethoscopes significantly enhance diagnostic accuracy, objectivity, and early detection. In pediatrics, AI improves heart murmur classification [], respiratory sound detection [,], and pneumonia identification in low-resource settings [], while supporting early intervention for neonatal respiratory distress []. In cardiology, AI-guided auscultation enables reliable detection of valvular disease [], peripartum cardiomyopathy [], and asymptomatic LVSD [], providing scalable, non-invasive screening that reduces dependence on echocardiography. In pulmonology, deep learning models applied to lung sounds, alone or with ultrasound, achieve high accuracy for chronic diseases such as ILD and COPD [] and allow rapid assessment of infectious diseases, including COVID-19 []. Overall, AI-powered digital auscultation decreases inter-observer variability, supports timely clinical decisions, and offers particular advantages in pediatric care and resource-limited environments.
AI-enhanced digital stethoscopes represent a paradigm shift in auscultation, converting a traditionally subjective skill into an objective diagnostic process. Evidence-based studies confirm their superiority in detecting pediatric murmurs, neonatal respiratory distress, valvular disease, and chronic pulmonary conditions (Table 2). Benefits include improved accuracy, timely interventions, reduced healthcare costs, and increased access in low-resource and remote settings.
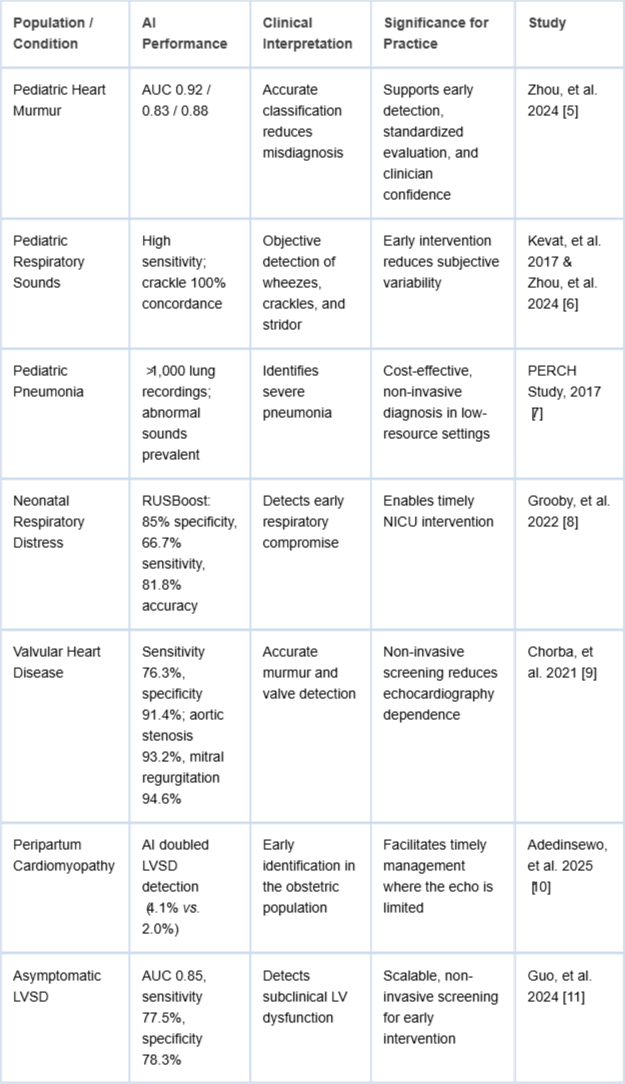 Table 2: Clinical Interpretation and Significance of evidence-based clinical studies in the era of artificial intelligence digital stethoscopes.
Table 2: Clinical Interpretation and Significance of evidence-based clinical studies in the era of artificial intelligence digital stethoscopes.Challenges remain, including clinician training, integration into existing workflows, and standardization of AI models across diverse populations. Adoption requires trust in algorithmic outputs, regulatory approval, and ongoing validation to ensure performance across age groups, comorbidities, and ethnicities. Moreover, data privacy, security, and interoperability must be addressed, particularly in telemedicine and IoMT-enabled systems [].
The COVID-19 pandemic further demonstrated the utility of digital stethoscopes in decentralized care. AI algorithms facilitated rapid triage and disease severity assessment, highlighting the potential for these devices in telehealth, pandemic preparedness, and resource-limited environments. As clinical datasets expand, algorithms will become increasingly sophisticated, potentially integrating multi-modal signals such as ECG, oximetry, and respiratory rate, to provide a holistic, patient-specific diagnostic output.
Emerging technologies include epidermal acoustic sensors and soft polymer piezoelectric devices for continuous, unobtrusive monitoring. These sensors offer high-fidelity sound capture without tubing interference, improved chest conformity, and wireless streaming to mobile and cloud platforms. Such devices are ideal for pediatric, neonatal, and long-term care applications [,].
Integration of heart sound analysis with ECG, oximetry, respiratory rate sensors, and motion detectors provides a more comprehensive clinical picture. Algorithms may detect compound pathologies, such as heart failure exacerbations, by analyzing simultaneous signals [].
AI can establish individualized acoustic baselines for patients, enabling detection of subtle trends in murmurs or wheezes. Personalized monitoring improves sensitivity, reduces false positives, and supports early detection of chronic conditions such as heart failure and asthma [].
Augmented reality can overlay auscultation data and diagnostic suggestions onto a clinician’s view, enhancing workflow efficiency, standardization, and documentation. Integration with EHRs ensures consistent recording and real-time decision support [].
Future digital stethoscopes will integrate with telehealth platforms and hospital systems. Compliance with standards such as FHIR, HL7, DICOM-WAVE, and IEEE 11073 will ensure secure data exchange, real-time streaming, and support predictive analytics, population health management, and AI-assisted triage [].
While promising, digital stethoscopes with AI face several limitations:
− Data quality and variability: Training algorithms require high-quality, annotated datasets. Variability in recording environments, patient movement, and background noise can impact model performance.
− Generalizability: Many studies are single-center or region-specific, raising questions about global applicability. Multi-center validation is necessary to ensure consistent performance across populations.
− Regulatory and ethical considerations: AI-guided diagnostics must comply with medical device regulations and privacy laws. Transparent algorithms and explainable AI are needed to support clinician trust.
− Cost and accessibility: Advanced digital stethoscopes may remain cost-prohibitive for some settings, particularly in low-income regions. Cost-effective solutions and scalable deployment models are critical.
− Integration with clinical workflows: For maximal impact, AI-powered stethoscopes must seamlessly integrate with EHRs, telemedicine platforms, and existing clinical protocols. Resistance to workflow changes may slow adoption.
Addressing these limitations will require collaborative efforts among engineers, clinicians, data scientists, and policymakers to ensure safe, effective, and equitable implementation.
The next generation of digital stethoscopes is expected to combine AI, flexible electronics, and multi-modal sensing to create smart, context-aware devices:
− Continuous monitoring: Wearable acoustic sensors will enable real-time monitoring of at-risk pediatric, neonatal, and cardiac patients.
− Personalized diagnostics: Longitudinal tracking of patient-specific acoustic profiles will allow early detection of subtle pathophysiological changes.
− Augmented decision support: Integration with AR, AI-guided interpretation, and predictive analytics will support clinicians at the point of care.
− Telemedicine expansion: IoMT-enabled stethoscopes will facilitate remote consultations, particularly in underserved regions.
− Multi-modal fusion: Combining heart and lung sounds with ECG, pulse oximetry, respiratory rate, and motion detection will allow a holistic assessment of cardiopulmonary status.
− Predictive analytics: Advanced AI algorithms may forecast disease progression, hospital readmissions, and acute exacerbations, guiding proactive interventions.
These innovations will not only improve diagnostic precision but also democratize access to high-quality care worldwide [].
AI-integrated digital stethoscopes represent a transformative advancement in evidence-based clinical practice. They enhance diagnostic accuracy, reduce inter-observer variability, and facilitate early detection of cardiac, pulmonary, and neonatal conditions. Recent studies highlight their utility in pediatric heart murmur classification, neonatal respiratory distress, peripartum cardiomyopathy, chronic lung disease, and COVID-19 management.
Future innovations, including wearable devices, sensor fusion, personalized acoustic profiles, and telemedicine integration, promise to expand their impact. Successful implementation will require robust datasets, regulatory oversight, clinician training, and seamless integration into clinical workflows. As the technology matures, AI-powered digital stethoscopes are poised to become indispensable tools, improving outcomes, expanding access, and shaping the future of patient-centered care.
No conflicts directly related to the content of this text. Professor E. Andrès has conducted fundamental and clinical research under institutional contracts with Alcatel-Lucent and the French National Technology Agency.
Andrès E, Gass R, Brandt C. State of the art on electronic stethoscopes in 2015. Med Ther. 2015;21:319–32.
Arjoune Y, Nguyen TN, Doroshow RW, Shekhar R. Technical characterisation of digital stethoscopes: towards scalable artificial intelligence-based auscultation. J Med Eng Technol. 2023 Apr;47(3):165–78. doi: 10.1080/03091902.2023.2174198. Epub 2023 Feb 15. PMID: 36794318; PMCID: PMC10753976.
Seah JJ, Zhao J, Wang Y, Lee HP. Review of the advancements of stethoscope types in chest auscultation. Diagnostics (Basel). 2023 Apr 25;13(9):1545. doi: 10.3390/diagnostics13091545. PMID: 37174938; PMCID: PMC10177339.
Kim Y, Hyon Y, Woo SD, Lee S, Lee SI, Ha T, Chung C. Evolution of the Stethoscope: Advances with the Adoption of Machine Learning and Development of Wearable Devices. Tuberc Respir Dis (Seoul). 2023 Oct;86(4):251-263. doi: 10.4046/trd.2023.0065. Epub 2023 Aug 18. PMID: 37592751; PMCID: PMC10555525.
Zhou G, Chien C, Chen J, Luan L, Chen Y, Carroll S, Dayton J, Thanjan M, Bayle K, Flynn P. Identifying pediatric heart murmurs and distinguishing innocent from pathologic using deep learning. Artif Intell Med. 2024 Jul;153:102867. doi: 10.1016/j.artmed.2024.102867. Epub 2024 Apr 4. PMID: 38723434.
Kevat AC, Kalirajah A, Roseby R. Digital stethoscopes compared to standard auscultation for detecting abnormal paediatric breath sounds. Eur J Pediatr. 2017 Jul;176(7):989-992. doi: 10.1007/s00431-017-2929-5. Epub 2017 May 16. PMID: 28508991.
McCollum ED, Park DE, Watson NL, Buck WC, Bunthi C, Devendra A, Ebruke BE, Elhilali M, Emmanouilidou D, Garcia-Prats AJ, Githinji L, Hossain L, Madhi SA, Moore DP, Mulindwa J, Olson D, Awori JO, Vandepitte WP, Verwey C, West JE, Knoll MD, O'Brien KL, Feikin DR, Hammitt LL. Listening panel agreement and characteristics of lung sounds digitally recorded from children aged 1-59 months enrolled in the Pneumonia Etiology Research for Child Health (PERCH) case-control study. BMJ Open Respir Res. 2017 Jun 30;4(1):e000193. doi: 10.1136/bmjresp-2017-000193. PMID: 28883927; PMCID: PMC5531306.
Grooby E, Sitaula C, Tan K, Zhou L, King A, Ramanathan A, Malhotra A, Dumont GA, Marzbanrad F. Prediction of Neonatal Respiratory Distress in Term Babies at Birth from Digital Stethoscope Recorded Chest Sounds. Annu Int Conf IEEE Eng Med Biol Soc. 2022 Jul;2022:4996-4999. doi: 10.1109/EMBC48229.2022.9871449. PMID: 36086631.
Chorba JS, Shapiro AM, Le L, Maidens J, Prince J, Pham S, Kanzawa MM, Barbosa DN, Currie C, Brooks C, White BE, Huskin A, Paek J, Geocaris J, Elnathan D, Ronquillo R, Kim R, Alam ZH, Mahadevan VS, Fuller SG, Stalker GW, Bravo SA, Jean D, Lee JJ, Gjergjindreaj M, Mihos CG, Forman ST, Venkatraman S, McCarthy PM, Thomas JD. Deep Learning Algorithm for Automated Cardiac Murmur Detection via a Digital Stethoscope Platform. J Am Heart Assoc. 2021 May 4;10(9):e019905. doi: 10.1161/JAHA.120.019905. Epub 2021 Apr 26. PMID: 33899504; PMCID: PMC8200722.
Adedinsewo DA, Morales-Lara AC, Afolabi BB, Kushimo OA, Mbakwem AC, Ibiyemi KF, Ogunmodede JA, Raji HO, Ringim SH, Habib AA, Hamza SM, Ogah OS, Obajimi G, Saanu OO, Jagun OE, Inofomoh FO, Adeolu T, Karaye KM, Gaya SA, Alfa I, Yohanna C, Venkatachalam KL, Dugan J, Yao X, Sledge HJ, Johnson PW, Wieczorek MA, Attia ZI, Phillips SD, Yamani MH, Tobah YB, Rose CH, Sharpe EE, Lopez-Jimenez F, Friedman PA, Noseworthy PA, Carter RE; SPEC-AI Nigeria Investigators. Artificial intelligence guided screening for cardiomyopathies in an obstetric population: a pragmatic randomized clinical trial. Nat Med. 2024 Oct;30(10):2897-2906. doi: 10.1038/s41591-024-03243-9. Epub 2024 Sep 2. Erratum in: Nat Med. 2025 May;31(5):1715. doi: 10.1038/s41591-025-03554-5. PMID: 39223284; PMCID: PMC11485252.
Guo L, Pressman GS, Kieu SN, Marrus SB, Mathew G, Prince J, Lastowski E, McDonough RV, Currie C, Tiwari U, Maidens JN, Al-Sudani H, Friend E, Padmanabhan D, Kumar P, Kersh E, Venkatraman S, Qamruddin S. Automated Detection of Reduced Ejection Fraction Using an ECG-Enabled Digital Stethoscope: A Large Cohort Validation. JACC Adv. 2025 Mar;4(3):101619. doi: 10.1016/j.jacadv.2025.101619. Epub 2025 Feb 20. Erratum in: JACC Adv. 2025 Aug;4(8):101940. doi: 10.1016/j.jacadv.2025.101940. PMID: 39983614; PMCID: PMC11891699.
Siebert JN, Hartley MA, Courvoisier DS, Salamin M, Robotham L, Doenz J, Barazzone-Argiroffo C, Gervaix A, Bridevaux PO. Deep learning diagnostic and severity-stratification for interstitial lung diseases and chronic obstructive pulmonary disease in digital lung auscultations and ultrasonography: clinical protocol for an observational case-control study. BMC Pulm Med. 2023 Jun 2;23(1):191. doi: 10.1186/s12890-022-02255-w. PMID: 37264374; PMCID: PMC10234685.
Glangetas A, Hartley MA, Cantais A, Courvoisier DS, Rivollet D, Shama DM, Perez A, Spechbach H, Trombert V, Bourquin S, Jaggi M, Barazzone-Argiroffo C, Gervaix A, Siebert JN. Deep learning diagnostic and risk-stratification pattern detection for COVID-19 in digital lung auscultations: clinical protocol for a case-control and prospective cohort study. BMC Pulm Med. 2021 Mar 24;21(1):103. doi: 10.1186/s12890-021-01467-w. PMID: 33761909; PMCID: PMC7988633.
Lakhe A, Sodhi I, Warrier J, Sinha V. Development of digital stethoscope for telemedicine. J Med Eng Technol. 2016;40(1):20-4. doi: 10.3109/03091902.2015.1116633. Epub 2016 Jan 5. PMID: 26728637.
Yilmaz G, Rapin M, Pessoa D, Rocha BM, de Sousa AM, Rusconi R, Carvalho P, Wacker J, Paiva RP, Chételat O. A Wearable Stethoscope for Long-Term Ambulatory Respiratory Health Monitoring. Sensors (Basel). 2020 Sep 8;20(18):5124. doi: 10.3390/s20185124. PMID: 32911861; PMCID: PMC7571051.
Duggan D, Sarana V, Factor A, Shelevytska V, Temko A, Popovici E. Towards a Wearable, High Precision, Multi-Functional Stethoscope. Annu Int Conf IEEE Eng Med Biol Soc. 2024 Jul;2024:1-4. doi: 10.1109/EMBC53108.2024.10781607. PMID: 40039438.
Kono Y, Miura K, Kasai H, Ito S, Asahina M, Tanabe M, Nomura Y, Nakaguchi T. Breath Measurement Method for Synchronized Reproduction of Biological Tones in an Augmented Reality Auscultation Training System. Sensors (Basel). 2024 Mar 1;24(5):1626. doi: 10.3390/s24051626. PMID: 38475162; PMCID: PMC10934352.
Das A, Adams K, Stoicoiu S, Kunhiabdullah S, Mathew A. Amplification of Heart Sounds Using Digital Stethoscope in Simulation-Based Neonatal Resuscitation. Am J Perinatol. 2024 May;41(S 01):e2485-e2488. doi: 10.1055/a-2121-8500. Epub 2023 Jul 3. PMID: 37399848.
Andres E, Hajjam AE. Digital Stethoscope in the Era of Artificial Intelligence: A Comprehensive Review in the Era of Evidence-based Clinical Studies. IgMin Res. November 08, 2025; 3(11): 395-400. IgMin ID: igmin320; DOI:10.61927/igmin320; Available at: igmin.link/p320
次のリンクを共有した人は、このコンテンツを読むことができます:
1Department of Internal Medicine, Hautepierre Hospital, Strasbourg University Hospitals, France
2Laboratory of Nanomedicine, Imaging and Therapeutics, University of Technology of Belfort-Montbéliard, France
Address Correspondence:
Emmanuel Andres, Professor, MD, PhD, Department of Internal Medicine, Hautepierre Hospital, Strasbourg University Hospitals, France, Email: [email protected]
How to cite this article:
Andres E, Hajjam AE. Digital Stethoscope in the Era of Artificial Intelligence: A Comprehensive Review in the Era of Evidence-based Clinical Studies. IgMin Res. November 08, 2025; 3(11): 395-400. IgMin ID: igmin320; DOI:10.61927/igmin320; Available at: igmin.link/p320
Copyright: 2025 Andres E, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
 Table 1: Recent Studies on Digital Stethoscopes in Cardiolo...
Table 1: Recent Studies on Digital Stethoscopes in Cardiolo...
 Table 2: Clinical Interpretation and Significance of eviden...
Table 2: Clinical Interpretation and Significance of eviden...
Andrès E, Gass R, Brandt C. State of the art on electronic stethoscopes in 2015. Med Ther. 2015;21:319–32.
Arjoune Y, Nguyen TN, Doroshow RW, Shekhar R. Technical characterisation of digital stethoscopes: towards scalable artificial intelligence-based auscultation. J Med Eng Technol. 2023 Apr;47(3):165–78. doi: 10.1080/03091902.2023.2174198. Epub 2023 Feb 15. PMID: 36794318; PMCID: PMC10753976.
Seah JJ, Zhao J, Wang Y, Lee HP. Review of the advancements of stethoscope types in chest auscultation. Diagnostics (Basel). 2023 Apr 25;13(9):1545. doi: 10.3390/diagnostics13091545. PMID: 37174938; PMCID: PMC10177339.
Kim Y, Hyon Y, Woo SD, Lee S, Lee SI, Ha T, Chung C. Evolution of the Stethoscope: Advances with the Adoption of Machine Learning and Development of Wearable Devices. Tuberc Respir Dis (Seoul). 2023 Oct;86(4):251-263. doi: 10.4046/trd.2023.0065. Epub 2023 Aug 18. PMID: 37592751; PMCID: PMC10555525.
Zhou G, Chien C, Chen J, Luan L, Chen Y, Carroll S, Dayton J, Thanjan M, Bayle K, Flynn P. Identifying pediatric heart murmurs and distinguishing innocent from pathologic using deep learning. Artif Intell Med. 2024 Jul;153:102867. doi: 10.1016/j.artmed.2024.102867. Epub 2024 Apr 4. PMID: 38723434.
Kevat AC, Kalirajah A, Roseby R. Digital stethoscopes compared to standard auscultation for detecting abnormal paediatric breath sounds. Eur J Pediatr. 2017 Jul;176(7):989-992. doi: 10.1007/s00431-017-2929-5. Epub 2017 May 16. PMID: 28508991.
McCollum ED, Park DE, Watson NL, Buck WC, Bunthi C, Devendra A, Ebruke BE, Elhilali M, Emmanouilidou D, Garcia-Prats AJ, Githinji L, Hossain L, Madhi SA, Moore DP, Mulindwa J, Olson D, Awori JO, Vandepitte WP, Verwey C, West JE, Knoll MD, O'Brien KL, Feikin DR, Hammitt LL. Listening panel agreement and characteristics of lung sounds digitally recorded from children aged 1-59 months enrolled in the Pneumonia Etiology Research for Child Health (PERCH) case-control study. BMJ Open Respir Res. 2017 Jun 30;4(1):e000193. doi: 10.1136/bmjresp-2017-000193. PMID: 28883927; PMCID: PMC5531306.
Grooby E, Sitaula C, Tan K, Zhou L, King A, Ramanathan A, Malhotra A, Dumont GA, Marzbanrad F. Prediction of Neonatal Respiratory Distress in Term Babies at Birth from Digital Stethoscope Recorded Chest Sounds. Annu Int Conf IEEE Eng Med Biol Soc. 2022 Jul;2022:4996-4999. doi: 10.1109/EMBC48229.2022.9871449. PMID: 36086631.
Chorba JS, Shapiro AM, Le L, Maidens J, Prince J, Pham S, Kanzawa MM, Barbosa DN, Currie C, Brooks C, White BE, Huskin A, Paek J, Geocaris J, Elnathan D, Ronquillo R, Kim R, Alam ZH, Mahadevan VS, Fuller SG, Stalker GW, Bravo SA, Jean D, Lee JJ, Gjergjindreaj M, Mihos CG, Forman ST, Venkatraman S, McCarthy PM, Thomas JD. Deep Learning Algorithm for Automated Cardiac Murmur Detection via a Digital Stethoscope Platform. J Am Heart Assoc. 2021 May 4;10(9):e019905. doi: 10.1161/JAHA.120.019905. Epub 2021 Apr 26. PMID: 33899504; PMCID: PMC8200722.
Adedinsewo DA, Morales-Lara AC, Afolabi BB, Kushimo OA, Mbakwem AC, Ibiyemi KF, Ogunmodede JA, Raji HO, Ringim SH, Habib AA, Hamza SM, Ogah OS, Obajimi G, Saanu OO, Jagun OE, Inofomoh FO, Adeolu T, Karaye KM, Gaya SA, Alfa I, Yohanna C, Venkatachalam KL, Dugan J, Yao X, Sledge HJ, Johnson PW, Wieczorek MA, Attia ZI, Phillips SD, Yamani MH, Tobah YB, Rose CH, Sharpe EE, Lopez-Jimenez F, Friedman PA, Noseworthy PA, Carter RE; SPEC-AI Nigeria Investigators. Artificial intelligence guided screening for cardiomyopathies in an obstetric population: a pragmatic randomized clinical trial. Nat Med. 2024 Oct;30(10):2897-2906. doi: 10.1038/s41591-024-03243-9. Epub 2024 Sep 2. Erratum in: Nat Med. 2025 May;31(5):1715. doi: 10.1038/s41591-025-03554-5. PMID: 39223284; PMCID: PMC11485252.
Guo L, Pressman GS, Kieu SN, Marrus SB, Mathew G, Prince J, Lastowski E, McDonough RV, Currie C, Tiwari U, Maidens JN, Al-Sudani H, Friend E, Padmanabhan D, Kumar P, Kersh E, Venkatraman S, Qamruddin S. Automated Detection of Reduced Ejection Fraction Using an ECG-Enabled Digital Stethoscope: A Large Cohort Validation. JACC Adv. 2025 Mar;4(3):101619. doi: 10.1016/j.jacadv.2025.101619. Epub 2025 Feb 20. Erratum in: JACC Adv. 2025 Aug;4(8):101940. doi: 10.1016/j.jacadv.2025.101940. PMID: 39983614; PMCID: PMC11891699.
Siebert JN, Hartley MA, Courvoisier DS, Salamin M, Robotham L, Doenz J, Barazzone-Argiroffo C, Gervaix A, Bridevaux PO. Deep learning diagnostic and severity-stratification for interstitial lung diseases and chronic obstructive pulmonary disease in digital lung auscultations and ultrasonography: clinical protocol for an observational case-control study. BMC Pulm Med. 2023 Jun 2;23(1):191. doi: 10.1186/s12890-022-02255-w. PMID: 37264374; PMCID: PMC10234685.
Glangetas A, Hartley MA, Cantais A, Courvoisier DS, Rivollet D, Shama DM, Perez A, Spechbach H, Trombert V, Bourquin S, Jaggi M, Barazzone-Argiroffo C, Gervaix A, Siebert JN. Deep learning diagnostic and risk-stratification pattern detection for COVID-19 in digital lung auscultations: clinical protocol for a case-control and prospective cohort study. BMC Pulm Med. 2021 Mar 24;21(1):103. doi: 10.1186/s12890-021-01467-w. PMID: 33761909; PMCID: PMC7988633.
Lakhe A, Sodhi I, Warrier J, Sinha V. Development of digital stethoscope for telemedicine. J Med Eng Technol. 2016;40(1):20-4. doi: 10.3109/03091902.2015.1116633. Epub 2016 Jan 5. PMID: 26728637.
Yilmaz G, Rapin M, Pessoa D, Rocha BM, de Sousa AM, Rusconi R, Carvalho P, Wacker J, Paiva RP, Chételat O. A Wearable Stethoscope for Long-Term Ambulatory Respiratory Health Monitoring. Sensors (Basel). 2020 Sep 8;20(18):5124. doi: 10.3390/s20185124. PMID: 32911861; PMCID: PMC7571051.
Duggan D, Sarana V, Factor A, Shelevytska V, Temko A, Popovici E. Towards a Wearable, High Precision, Multi-Functional Stethoscope. Annu Int Conf IEEE Eng Med Biol Soc. 2024 Jul;2024:1-4. doi: 10.1109/EMBC53108.2024.10781607. PMID: 40039438.
Kono Y, Miura K, Kasai H, Ito S, Asahina M, Tanabe M, Nomura Y, Nakaguchi T. Breath Measurement Method for Synchronized Reproduction of Biological Tones in an Augmented Reality Auscultation Training System. Sensors (Basel). 2024 Mar 1;24(5):1626. doi: 10.3390/s24051626. PMID: 38475162; PMCID: PMC10934352.
Das A, Adams K, Stoicoiu S, Kunhiabdullah S, Mathew A. Amplification of Heart Sounds Using Digital Stethoscope in Simulation-Based Neonatal Resuscitation. Am J Perinatol. 2024 May;41(S 01):e2485-e2488. doi: 10.1055/a-2121-8500. Epub 2023 Jul 3. PMID: 37399848.